Welcome to inquire:177-2247-7738
Shenzhen Headquarters: 4th Floor, No. 240, Third Cotton Tree Road, Longgang District, Shenzhen City, Guangdong Province
Anhui Production Base: 4th Floor, Building A1, Jiangzhi South Incubation Science Park, Guichi District, Chizhou City, Anhui Province
Jiangsu Branch: No. 3528 Xixi East Avenue, Anzhen Town, Xishan District, Wuxi City, Jiangsu Province
Zhejiang Lake Branch: 2nd Floor, Hongyuan Municipal, Changhong Road, Changxing County, Huzhou City, Zhejiang Province
Chongqing Branch: No. 108, Zone C, Jinke Electromechanical City, Jiulongpo District, Chongqing

Official wechat service number

Official video number

Official Douyin number
Products & Solutions
Lithium battery PACK production line
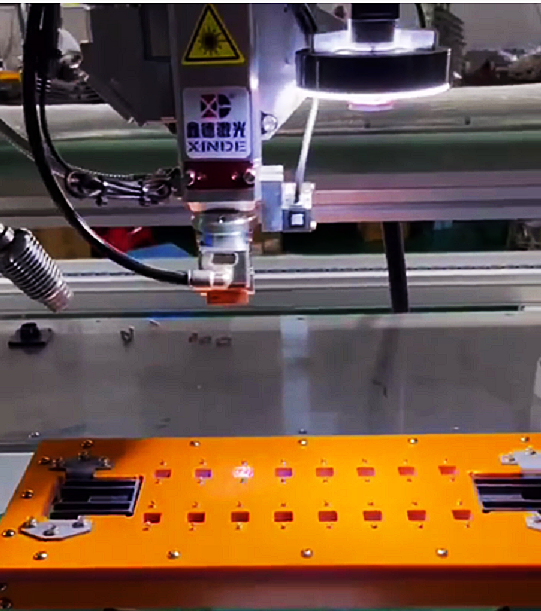
Lithium battery laser welding machine
Six axis manipulator laser welding machine
The Solution
Copyright © 2022 Xinde (Shenzhen) Laser Equipment Co, LTD All Rights Reserved. 粤ICP备18130442号